CoAuthor™ is Certara’s innovative GenAI software for regulatory and medical writing, designed to streamline the drafting of regulatory documents. By leveraging a leading-edge GPT, a comprehensive eCTD template library, and structured content authoring, CoAuthor empowers regulatory writing teams to focus on contextualizing data and developing key messages. With built-in transparency, consistency, and collaboration tools, CoAuthor reduces submission timelines, enabling faster access to life-saving medicines for patients.
CoAuthor
Meet your team’s newest regulatory writing assistant
Discover how CoAuthor can transform your regulatory and medical writing processes.
Transforming regulatory writing with CoAuthor
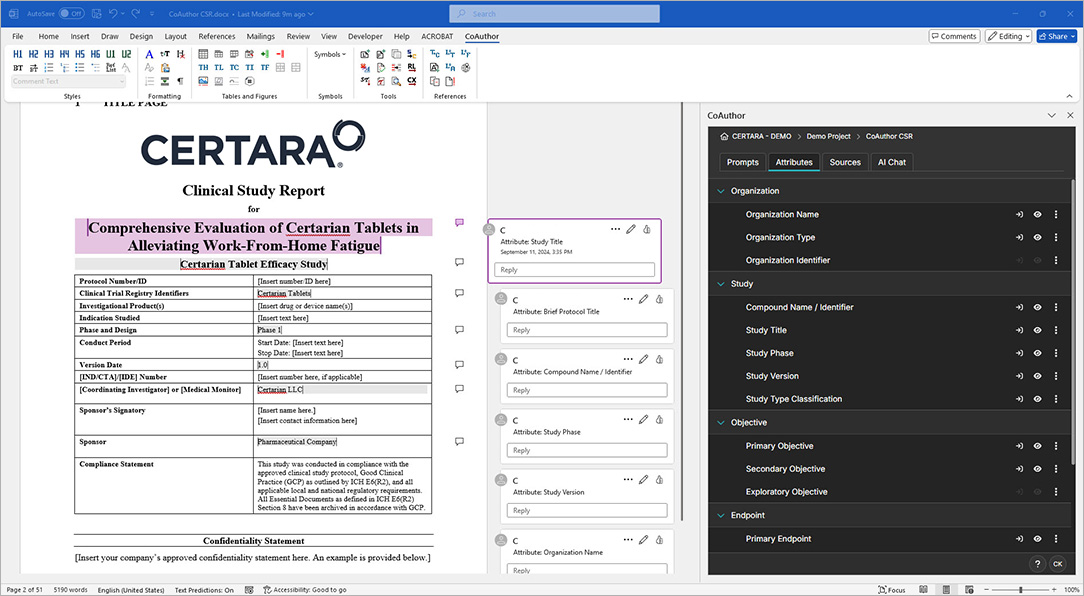
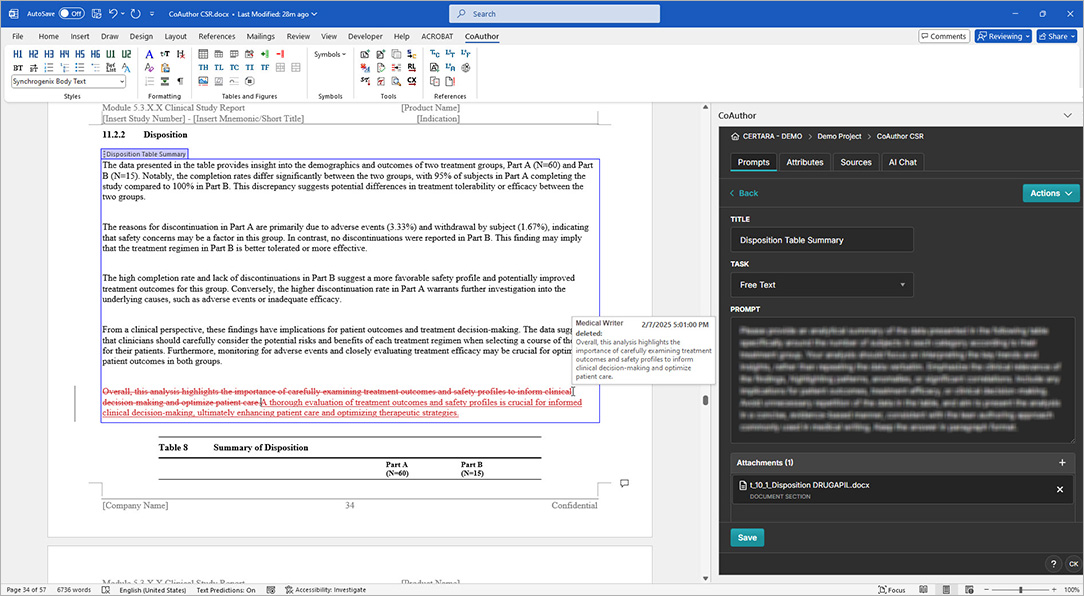
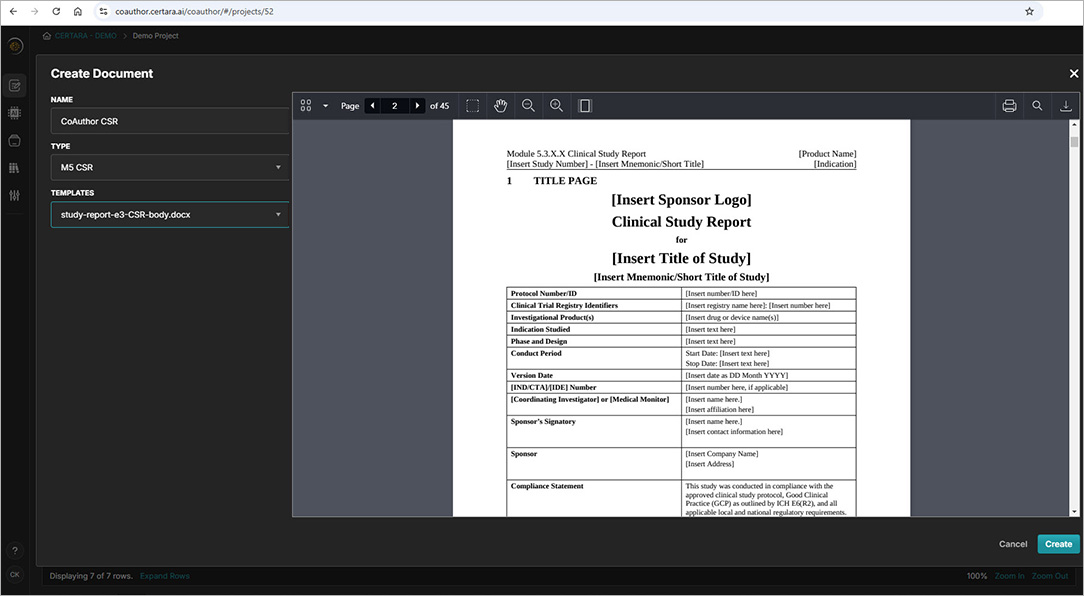
CoAuthor simplifies the complexities of regulatory writing by integrating a purpose-built biomedical GPT, structured content, and eCTD templates directly into Microsoft Word. This specialized GenAI software for medical writing ensures secure, organization-specific use, eliminating data leakage risks and enhancing document quality.
- Generate documents such as patient narratives, clinical study reports, and protocols with ease
- Leverage structured content authoring for content reuse and consistency
- Automate formatting, hyperlinking, and metadata population for efficiency
Write less, analyze more
CoAuthor unites all the features missing from off-the-shelf text AI and other regulatory tools
Regulatory document template suite
Structured content authoring
Regulatory-specific Generative AI
Auto styling & hyperlinking
Traceability & version control
Real time preview
Collaborative authoring & review
Meta-data & document repository
Off the shelf prompt library
MS Word integration
Regulatory document repository
Tech-enabled medical writing services
Related resources
View all
Your data is safe with CoAuthor
At Certara, we understand the paramount importance of protecting your information. That’s why we are proud to hold an ISO 27001 certification for Certara’s Information Security Management System (ISMS). With ISO 27001, you can have peace of mind knowing that we have implemented robust security controls, undergone rigorous risk assessments, and continuously strive for improvement.
Experience the future of regulatory writing with CoAuthor
Book a no-obligation demo to see how CoAuthor can revolutionize your regulatory writing processes.
FAQs
What types of documents can CoAuthor generate?
CoAuthor can be used to create patient narratives, clinical study reports, protocols, synopsis, toxicology reports, and and other clinical documents.
How does CoAuthor ensure data security?
CoAuthor is delivered as a secure, organization-specific model, eliminating risks of data leakage or exposure to public tools.
Can CoAuthor integrate with existing workflows?
Yes, CoAuthor integrates seamlessly with Microsoft Word and supports structured content authoring for streamlined workflows.
How do I write an effective prompt within CoAuthor?
A prompt has four basic parts: Context, Data, Task, and Format. Depending on your use case, you may not need all the different parts in your prompt, nor do they have to be in any particular order. A key part of prompt engineering is figuring out which parts of a prompt are needed and crafting them to get the response you want. Read our blog on Best Practices for AI Prompt Engineering in Life Sciences to find out more.