July 25, 2025
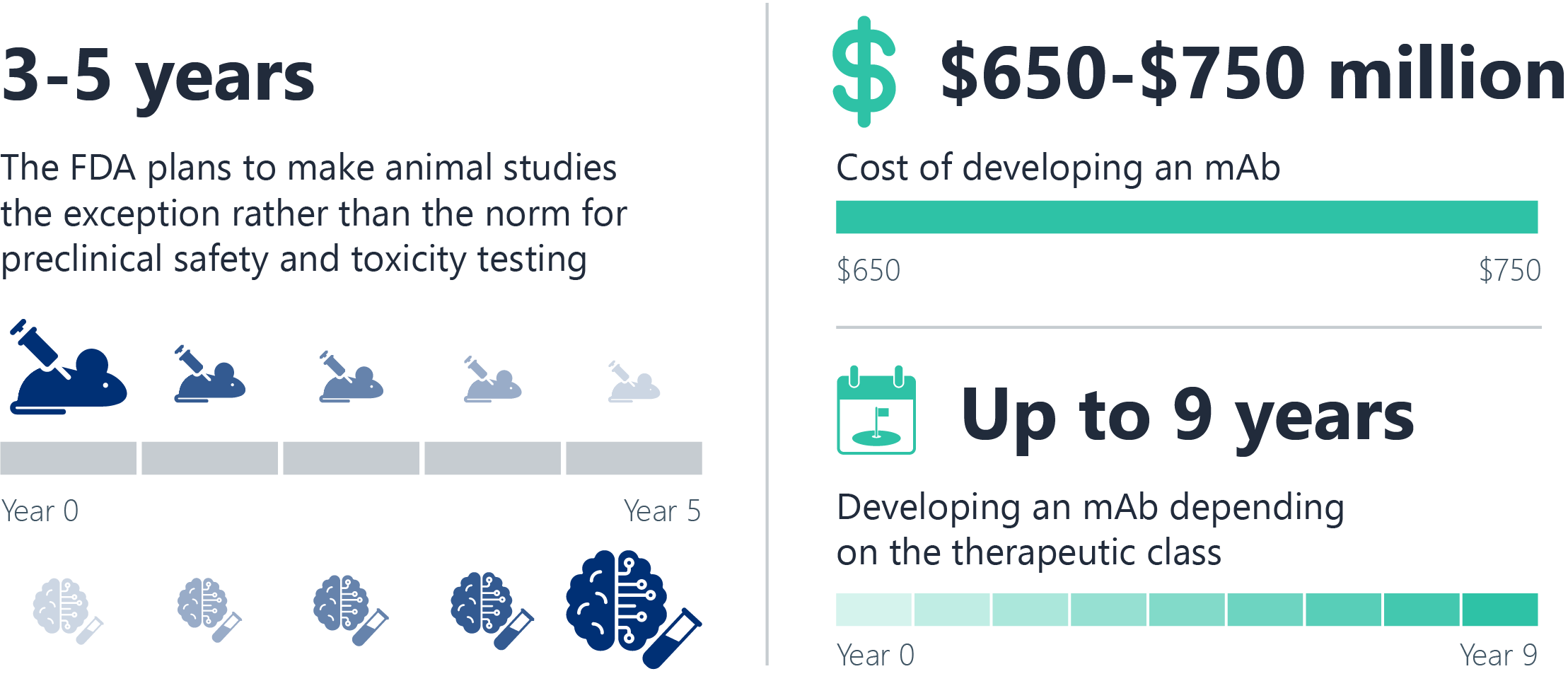
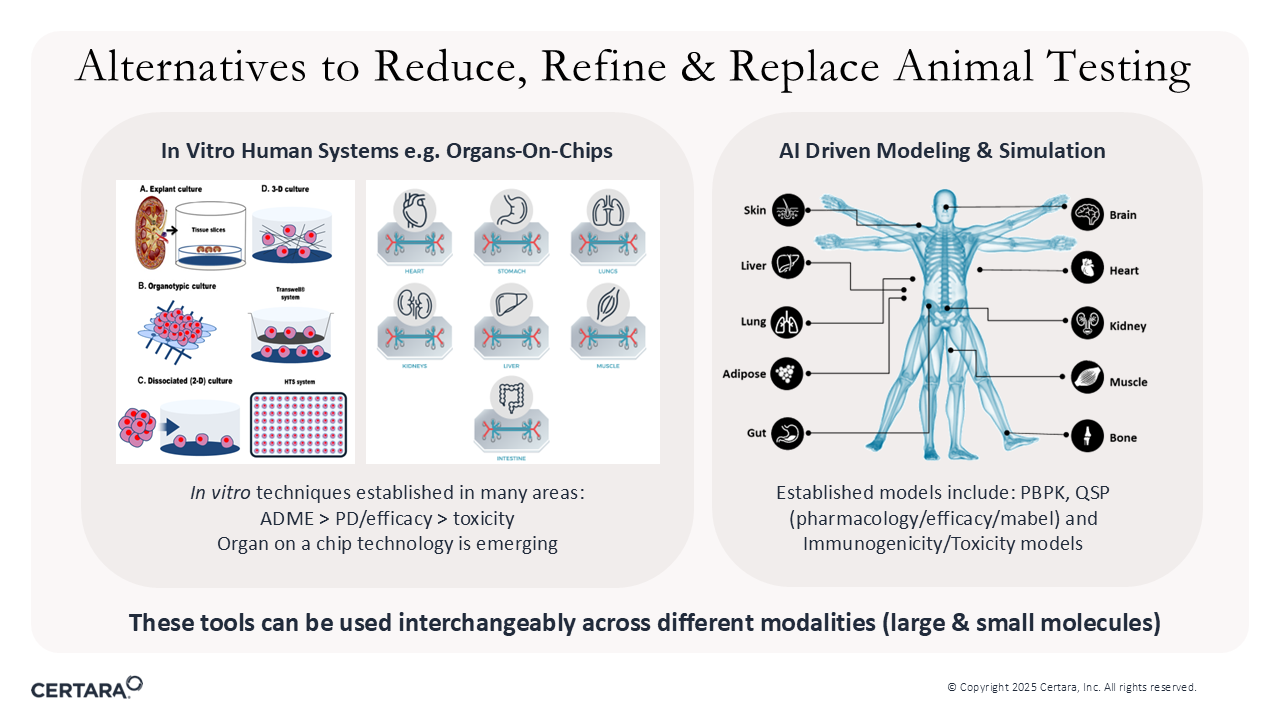
Figure 1. NAMs are alternatives to reduce, refine, and replace animal testing.
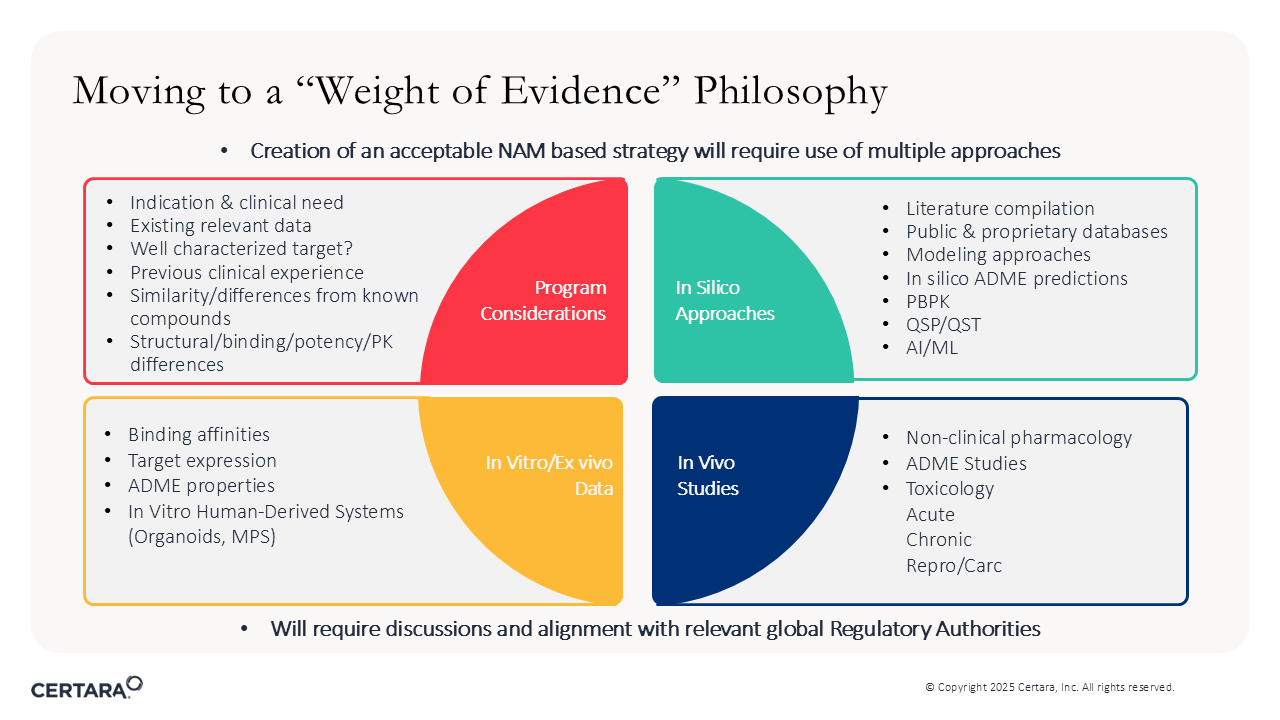
Figure 2. Drug developers must use a weight-of-evidence approach to develop their regulatory submissions for health authorities.
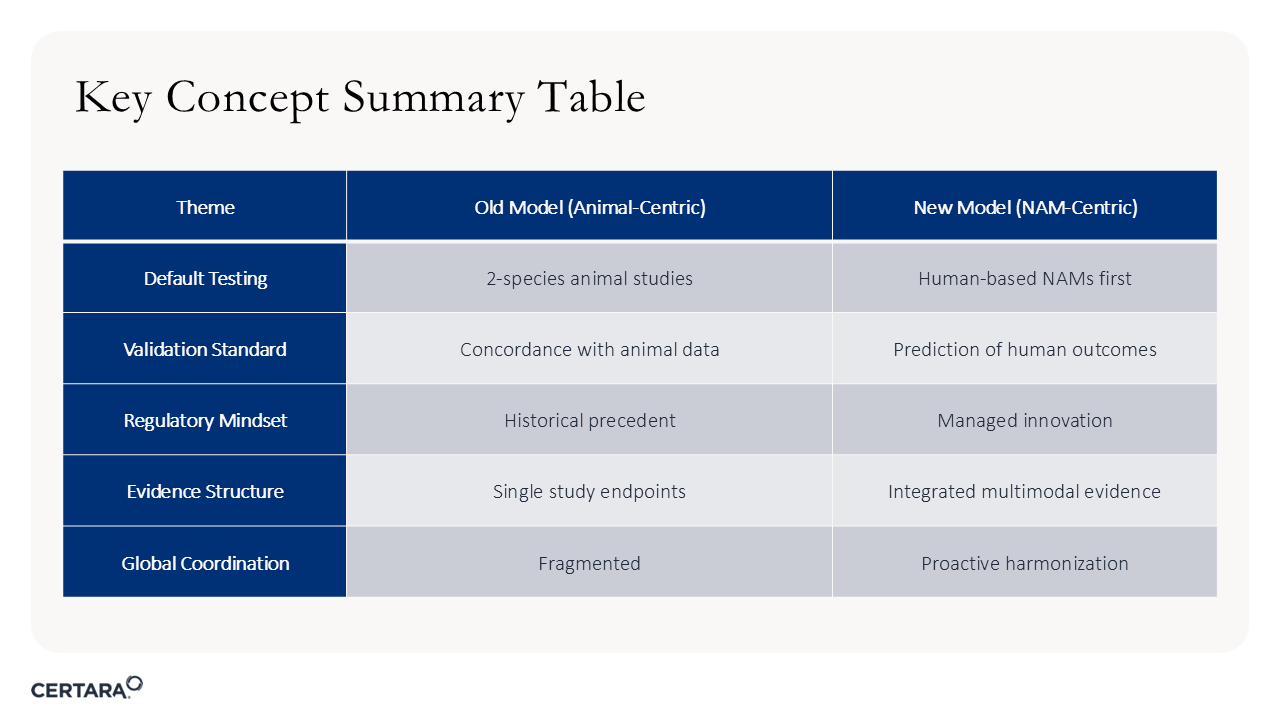
Figure 3. Shifting from animal-centric drug development to a NAM-centric model requires multiple considerations.
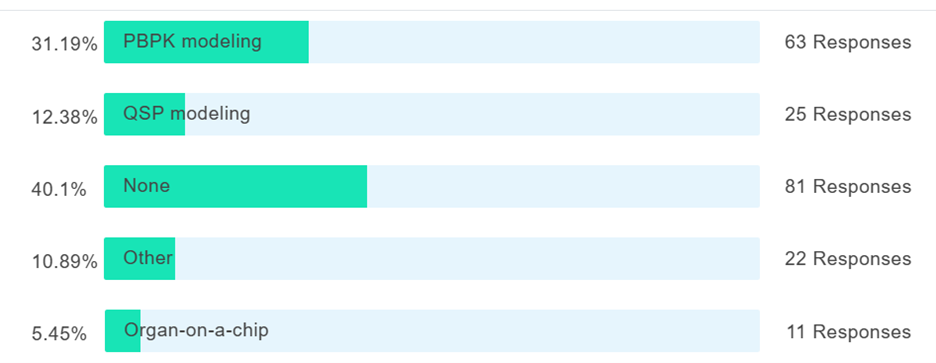
Figure 4. What type of New Approach Methodology have you used in preclinical drug development programs? N=200

Are you prepared for the FDA’s phase out of animal tests for mAbs?
The FDA’s plan to phase out animal testing is a transformative step that paves the way for innovative, human-relevant preclinical approaches that are more predictive, efficient, and ethical. There is a long history at the FDA of using validated new approach methodologies (NAMs), including in silico tools and computational modeling as a framework for regulatory decision-making to support this transition effectively.
Navigate the evolving nonclinical landscape with confidence
Certara’s Non-Animal Navigator™ solution helps companies adapt by selecting and optimizing the best-fit NAM strategies.


Director of Content Strategy, Certara
Dr. Suzanne Minton is the Director of Content Strategy where she leads a team of writers that develop the whip smart, educational, and persuasive content is the foundation of Certara’s thought leadership programs. She has a decade of experience in corporate marketing and has conducted biomedical research in infectious disease, cancer, pharmacology, and neurobiology. Suzanne earned a BS in biology from Duke University and a doctorate in pharmacology from the University of North Carolina at Chapel Hill.
Contact our expert consultants