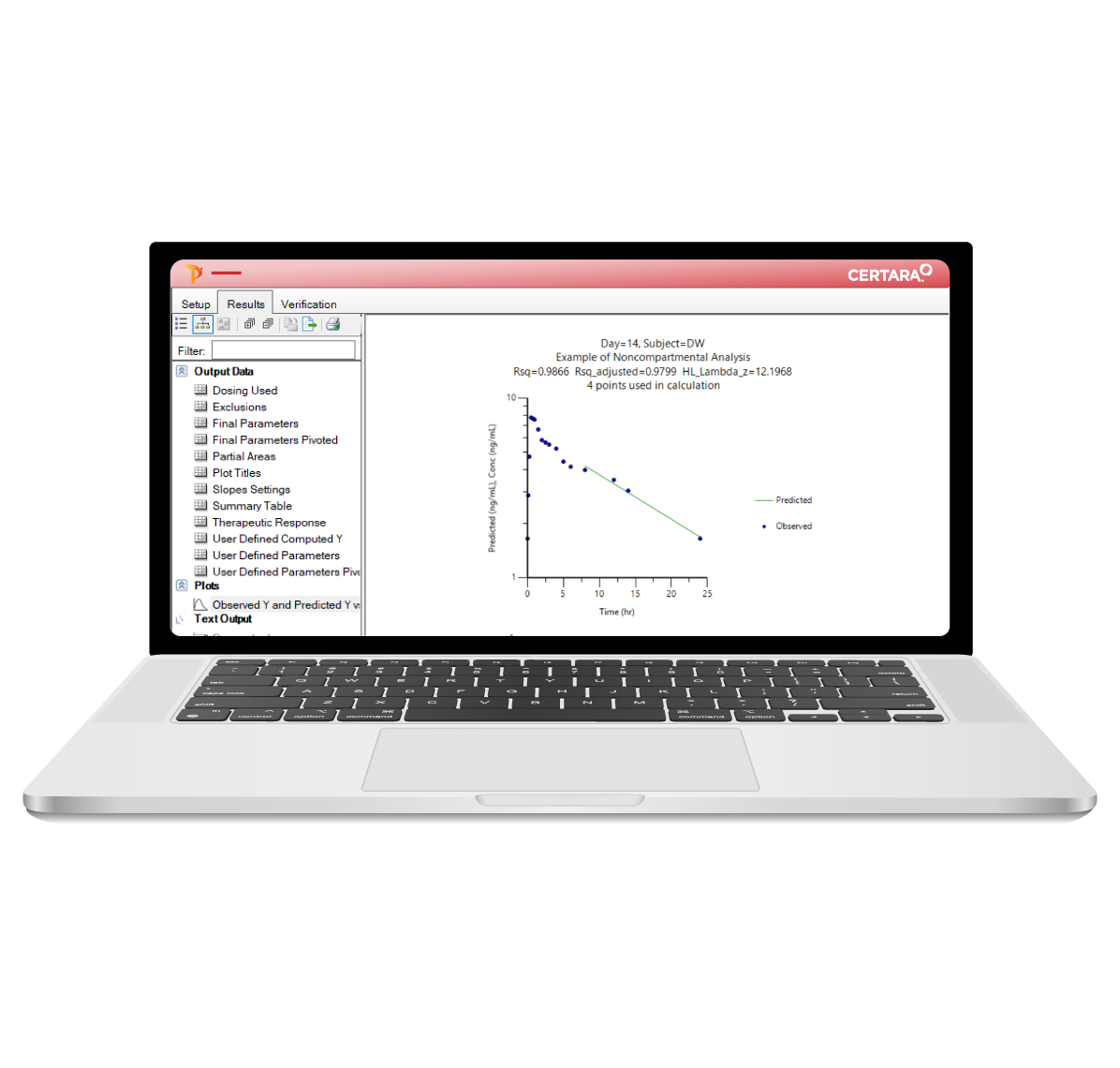
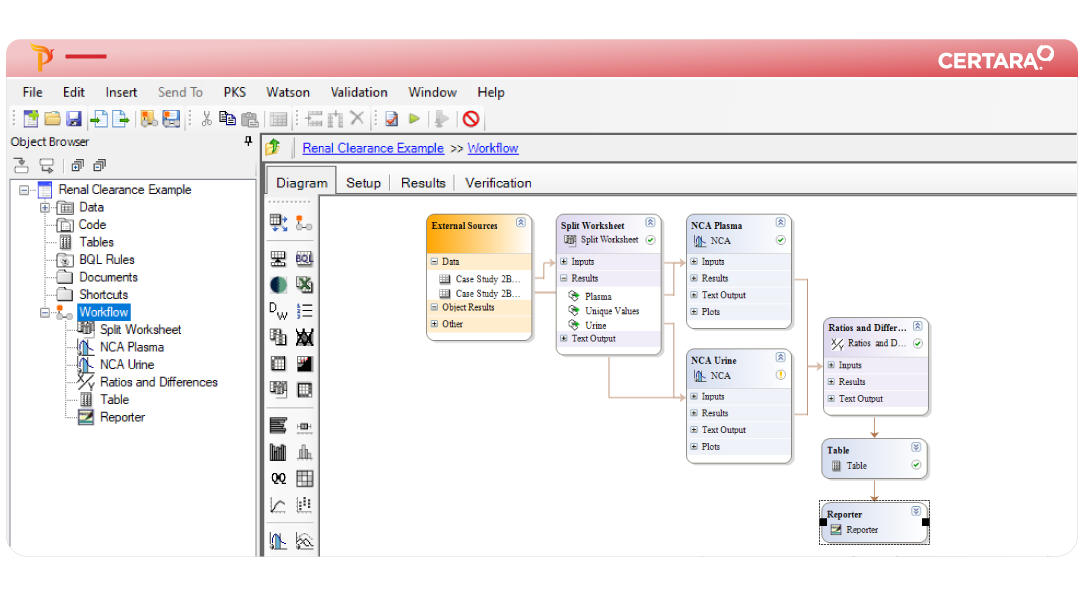
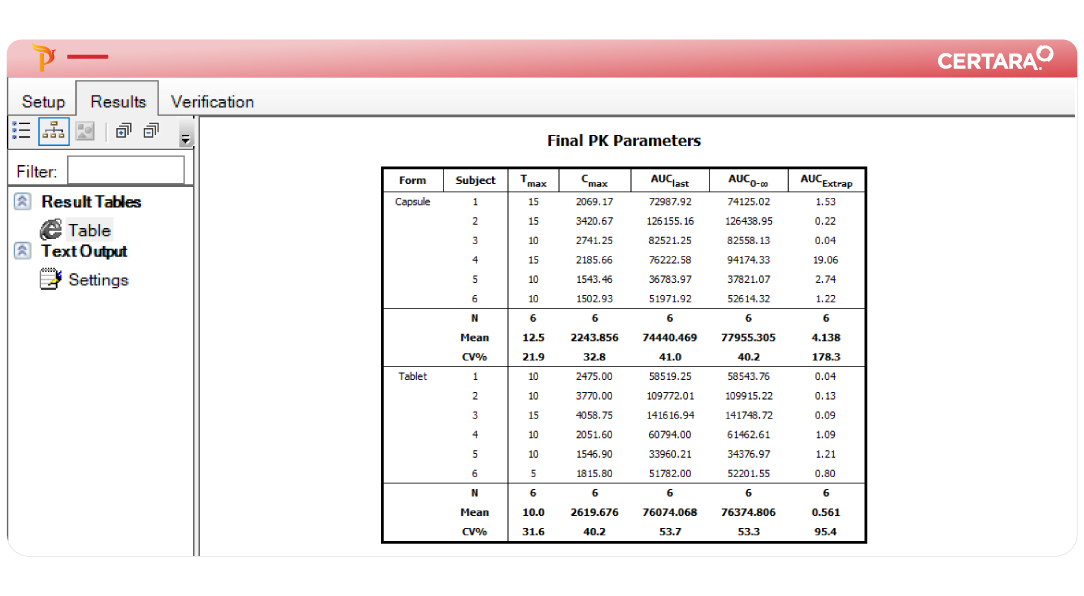
PK/PD and NCA can be time-consuming, requiring careful attention at every step – from data import to report generation. Phoenix WinNonlin® streamlines these processes with reproducible workflows, enabling collaborative and compliant PK/PD at scale.
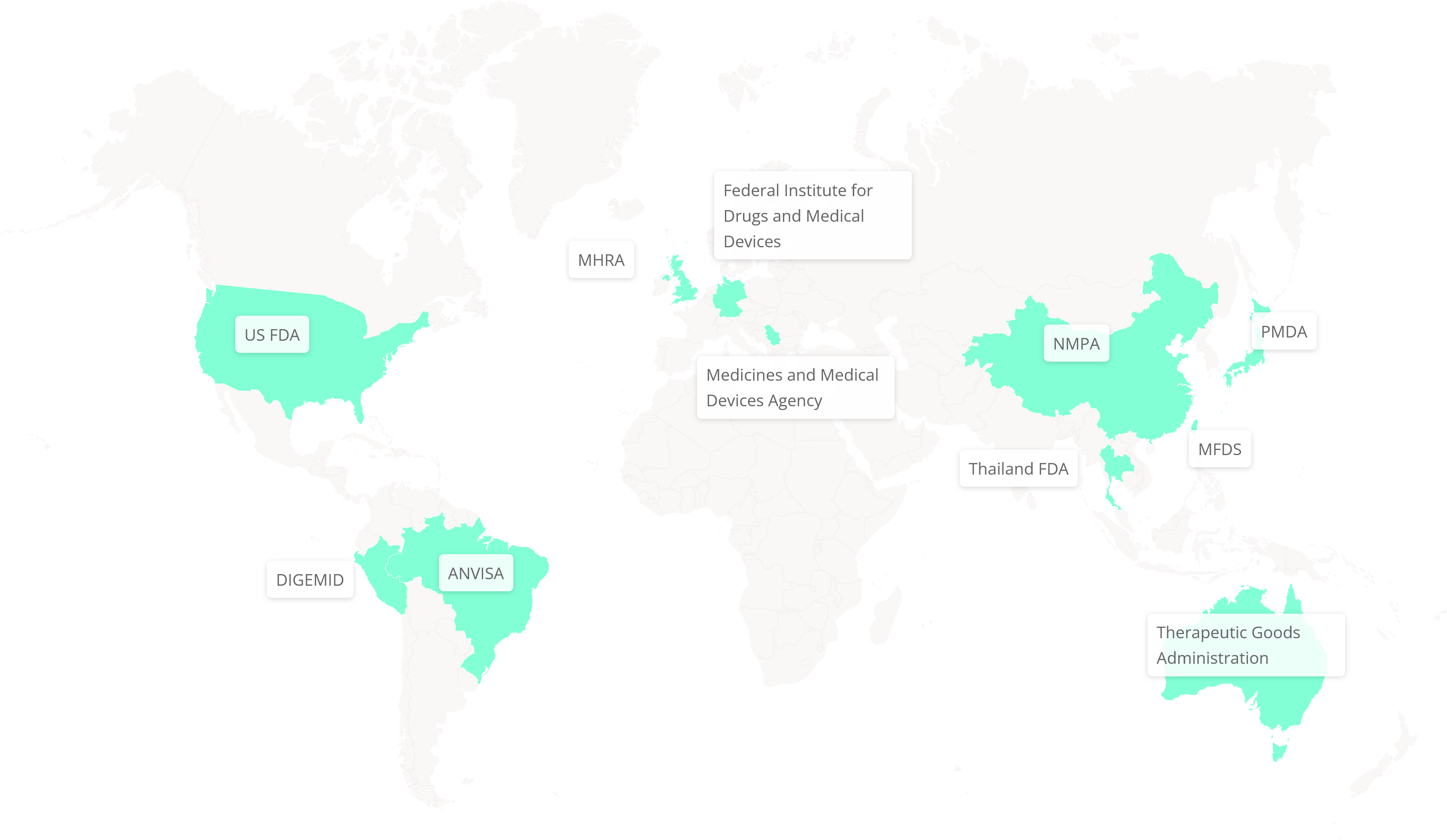
As the industry standard for NCA, PK/PD studies, and toxicokinetic (TK) modeling, Phoenix WinNonlin delivers 30 years of reliability and innovation. Trusted by regulatory agencies like the FDA, PMDA, CFDA, and MHRA, it ensures compliance, reduces manual effort, and enhances productivity.