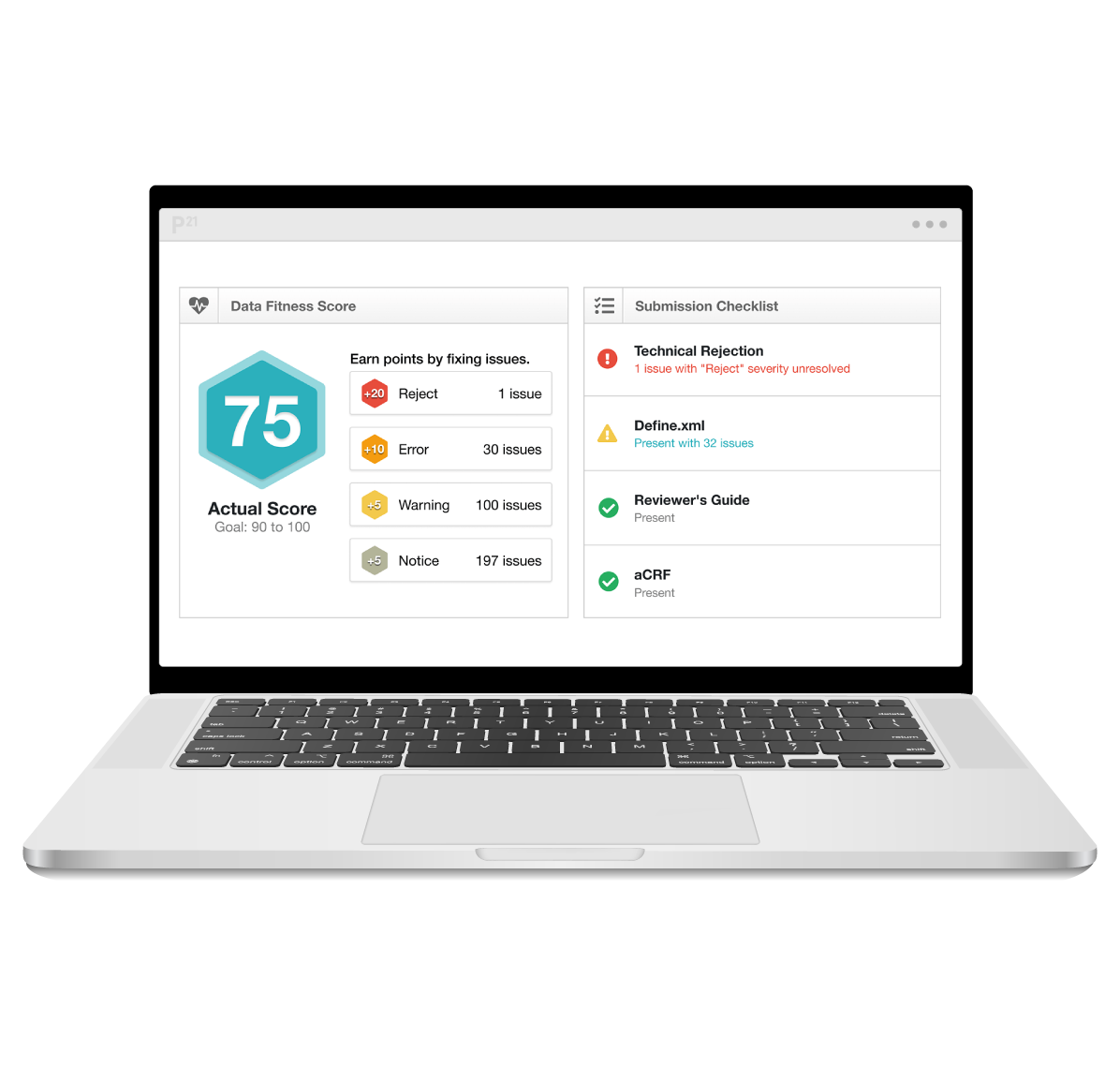
Let our clinical metadata repository transform your clinical data into high-quality, submission-ready deliverables. By combining metadata management and continuous data validation in a collaborative platform, you’ll accelerate timelines, reduce the risk of costly delays, and ensure end-to-end compliance.
Pinnacle 21®
Personalized demo of Pinnacle 21 clinical data standardization software
Driving results for leading pharma organizations




About Certara
Certara is dedicated to transforming drug discovery and development for good. We harness the power of biosimulation, advanced analytics, and regulatory expertise to create a future where treatments reach patients faster and more efficiently.
From discovery to market access and commercial, we tailor solutions to meet our clients’ most pressing challenges. Through strategic leadership and advanced predictive technologies, Certara provides comprehensive solutions to optimize drug development processes, reduce risks, and improve outcomes. Our clients include more than 2,400 biopharmaceutical companies, academic institutions, and regulatory agencies across 70 countries.
© 2025 Certara. All Rights Reserved. | Privacy policy