See how to achieve faster drug development, with less effort, and less manual work. The Pinnacle 21 software suite augments your capabilities – helping you quickly build validated studies, get cleaner vendor data (minus the delays), automate SDTM, and speed time-to-analysis. Powered by technology, you’ll be able to extract data insights earlier, and increase the speed and quality of submission deliverables.
Pinnacle 21
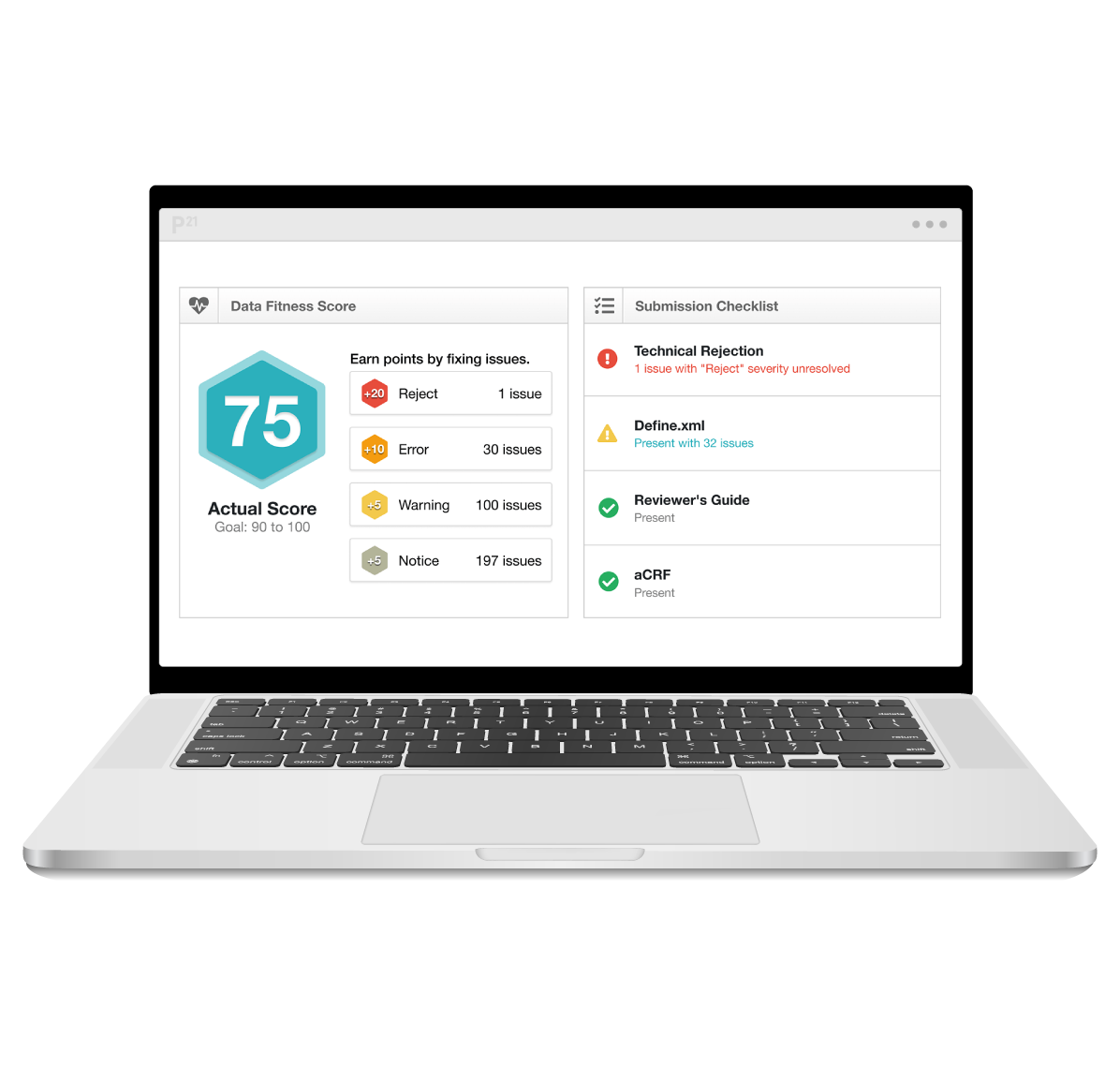
Build validated studies up to 85% faster
Build compliant studies faster & make earlier informed decisions
Build studies in under 6 weeks
Standardize and reuse content, saving time & effort on study setup.
Design and visualize CRFs
See how CRFs look and work for your chosen EDC – as you’re designing forms in Pinnacle 21.
Improve data quality & compliance
Increase quality with real-time validation and issue resolution, all in one central platform.
Get cleaner vendor data
Reduce data delivery timelines and get compliant data from vendors.
Transform clinical data with Pinnacle 21
Schedule a demo of Pinnacle 21 Enterprise
Schedule a demo or consultation to see how Pinnacle 21 can accelerate your path from study design to submission.
Master data standardization and validation
Experience a new dimension of submission efficiency
Harmonize data from diverse sources
